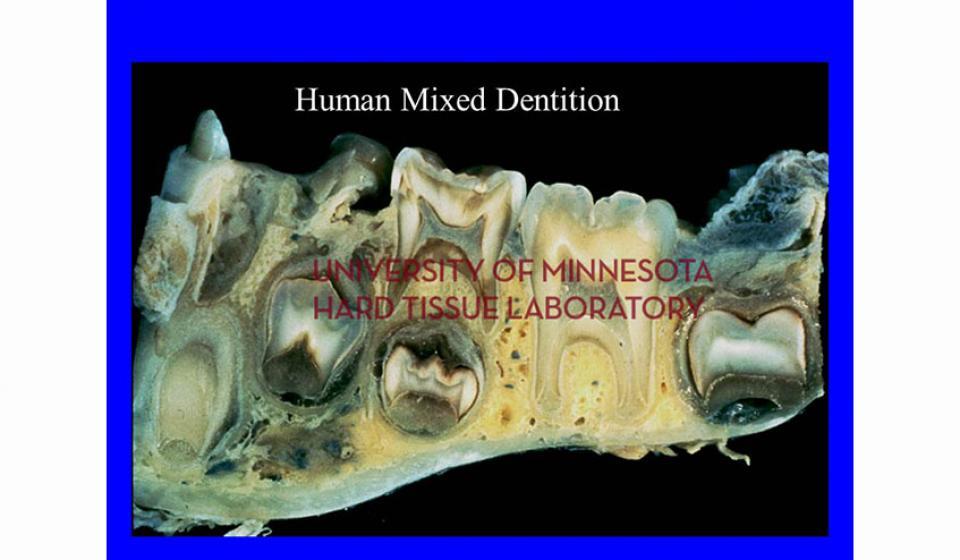
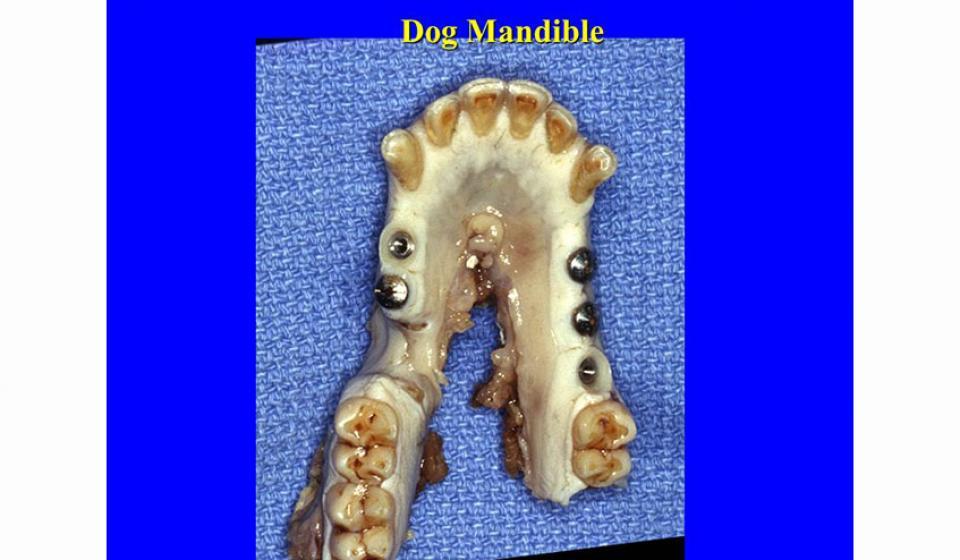
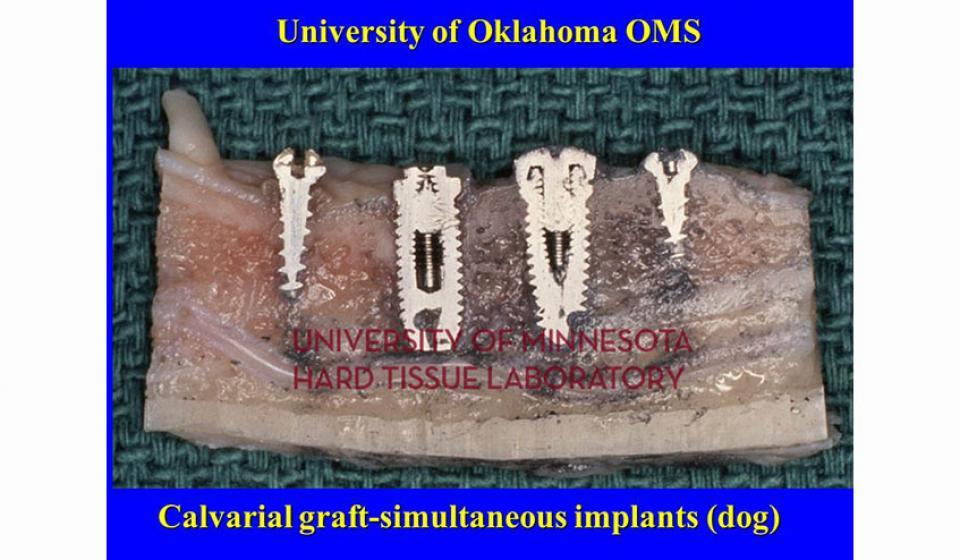
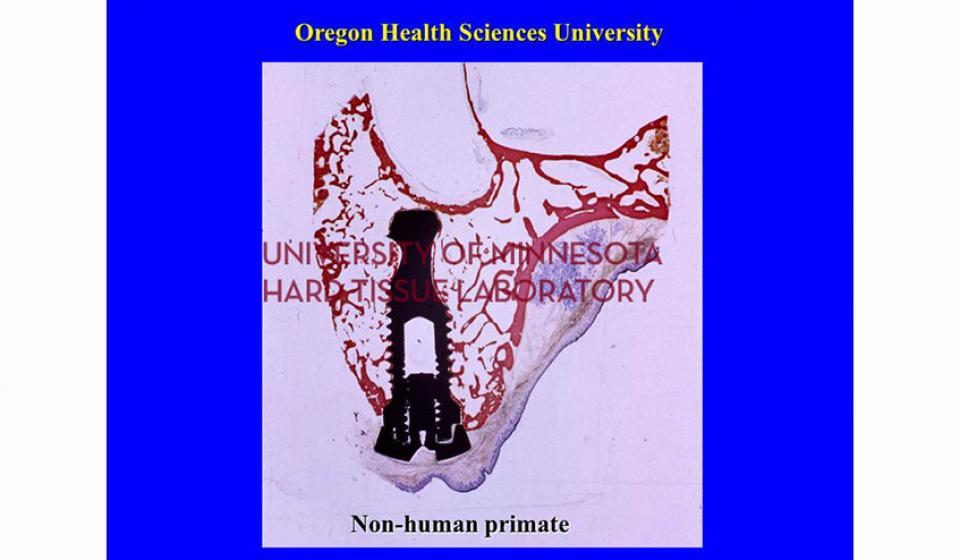
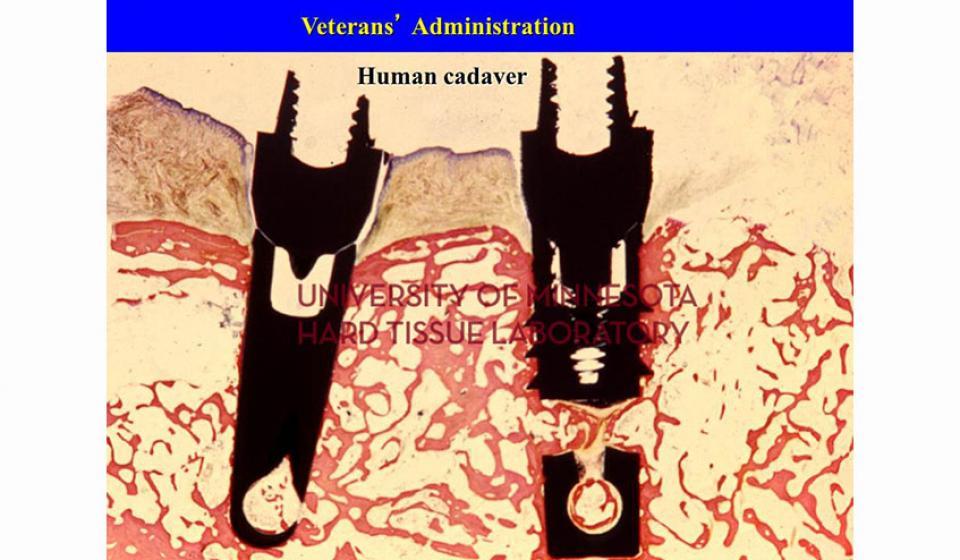
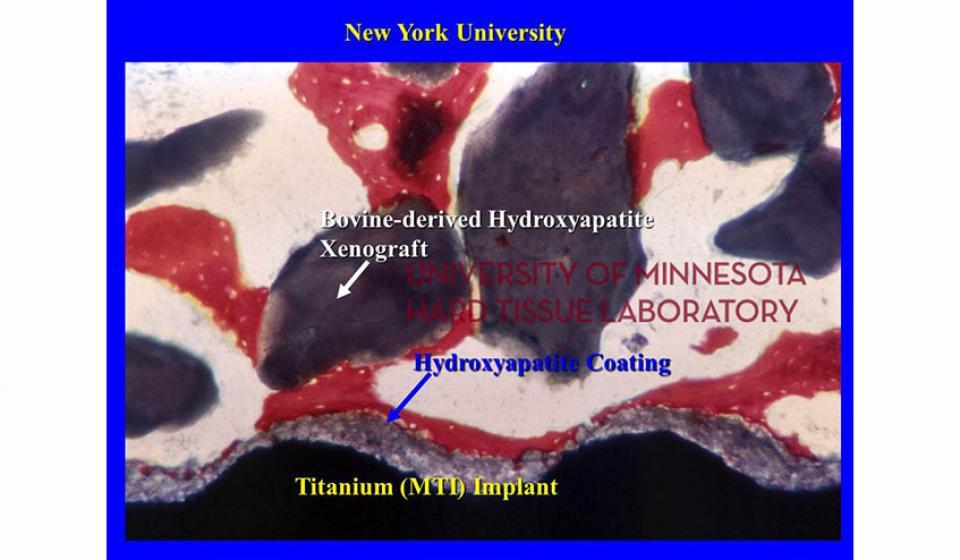
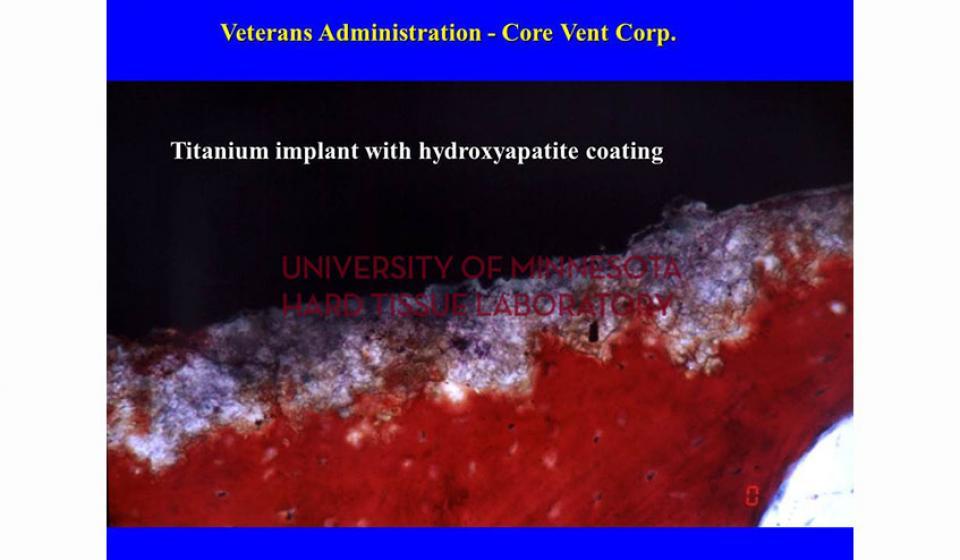
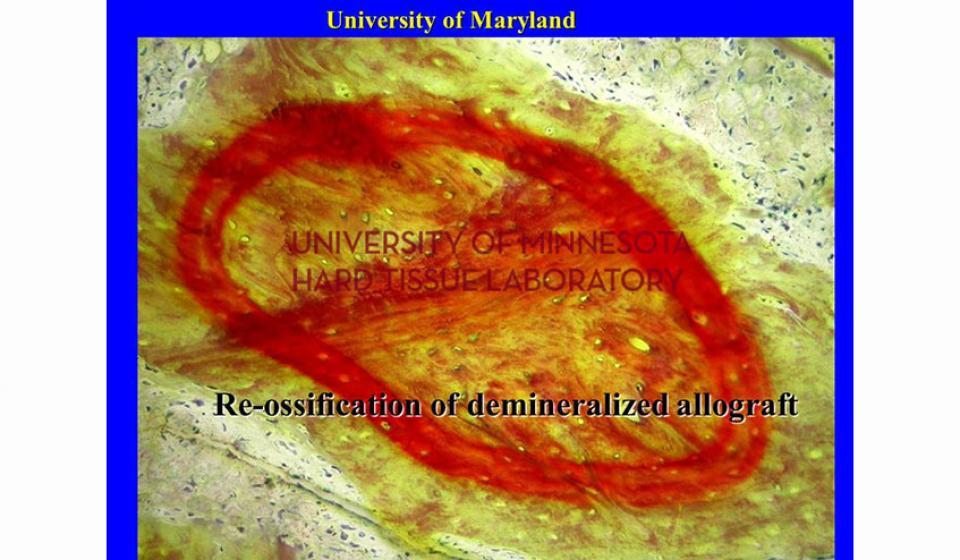
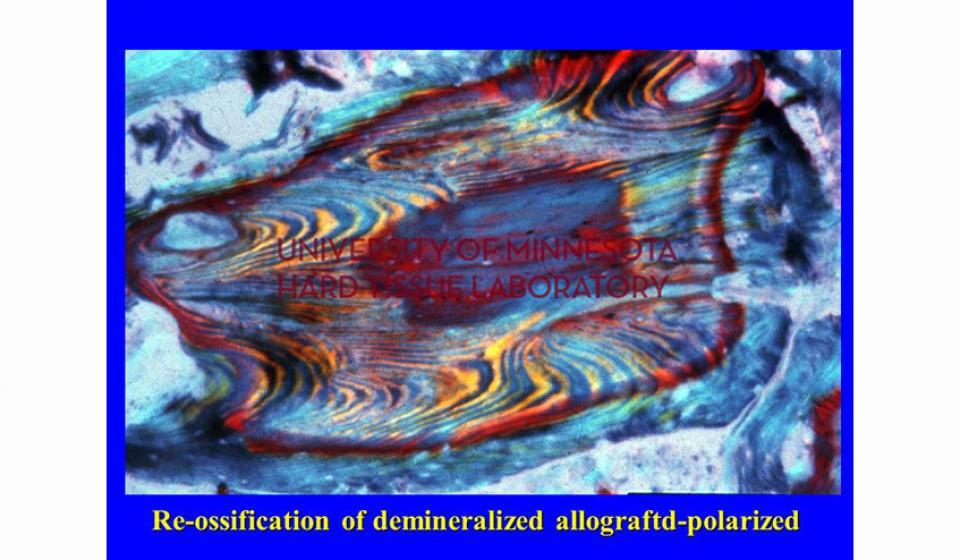
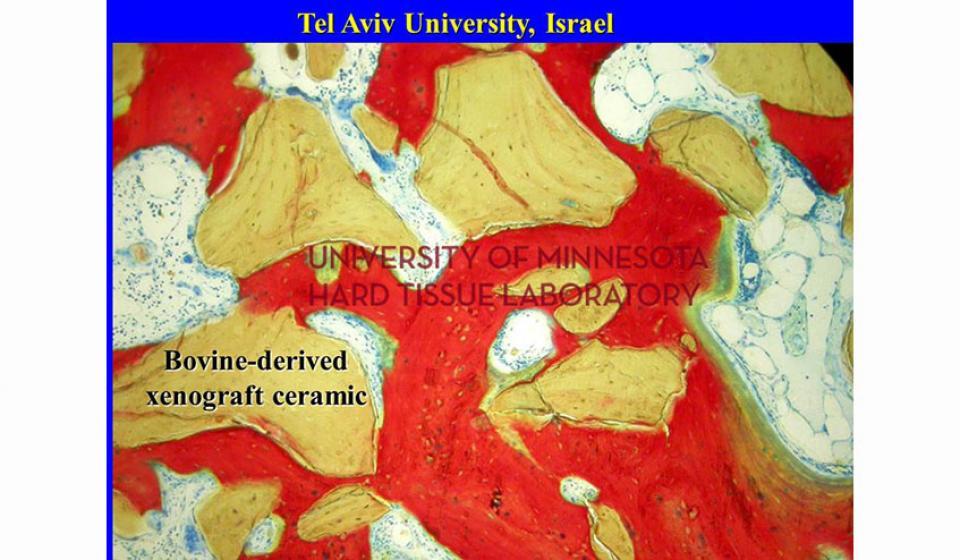
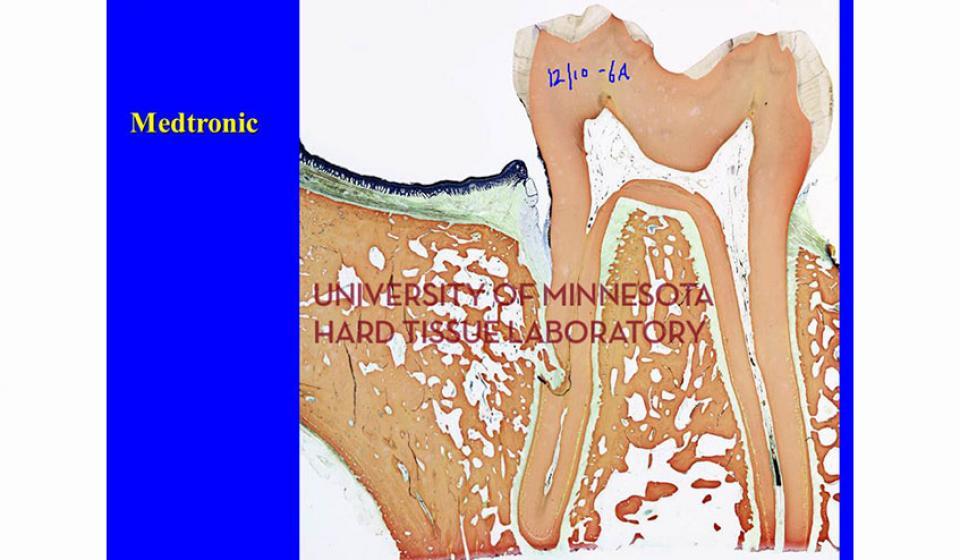
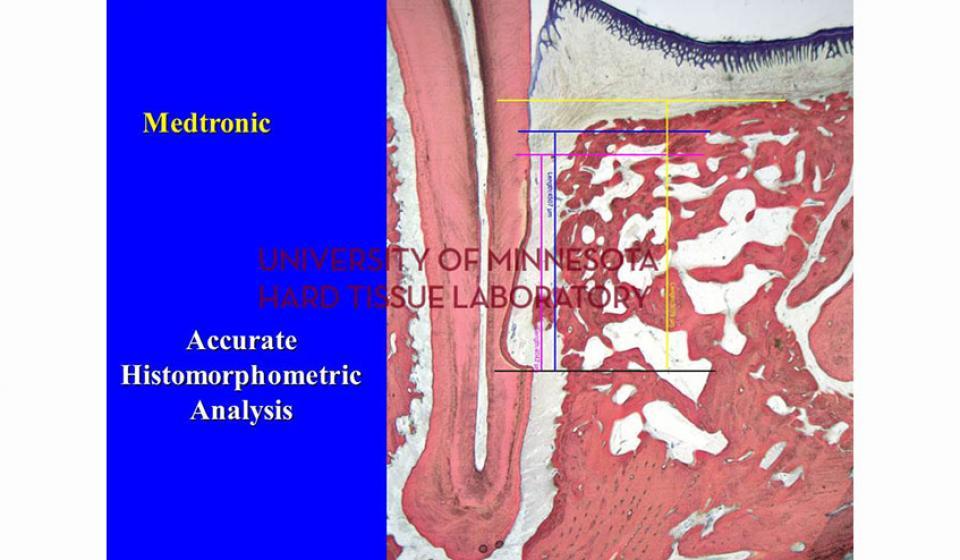
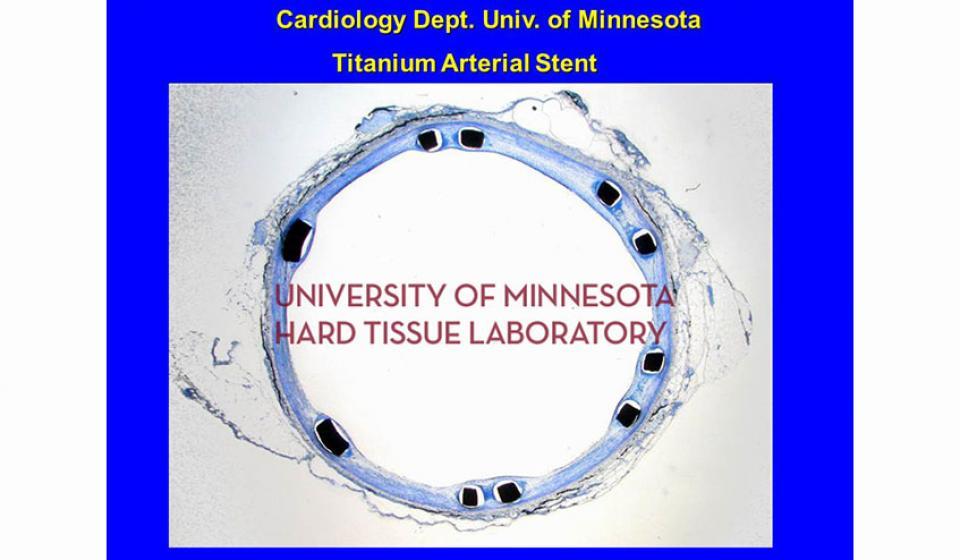
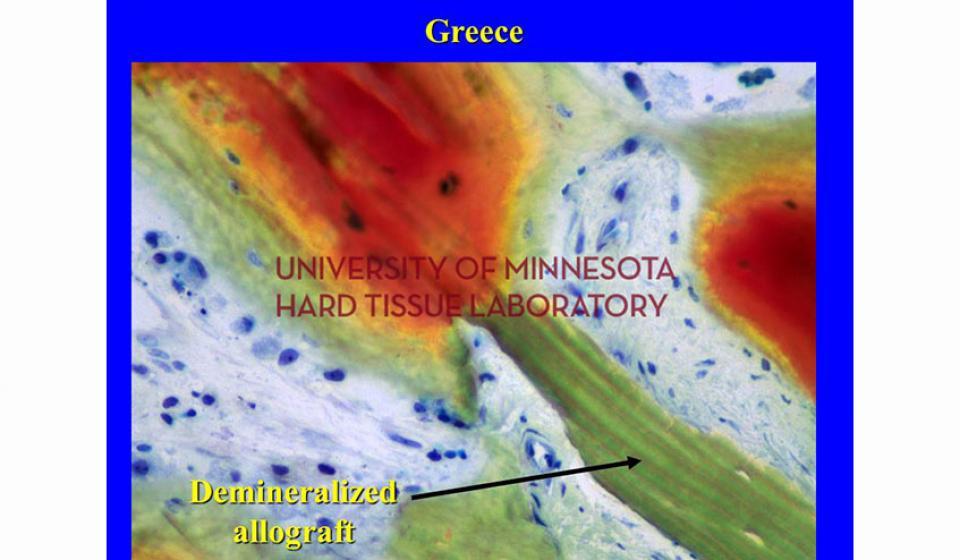
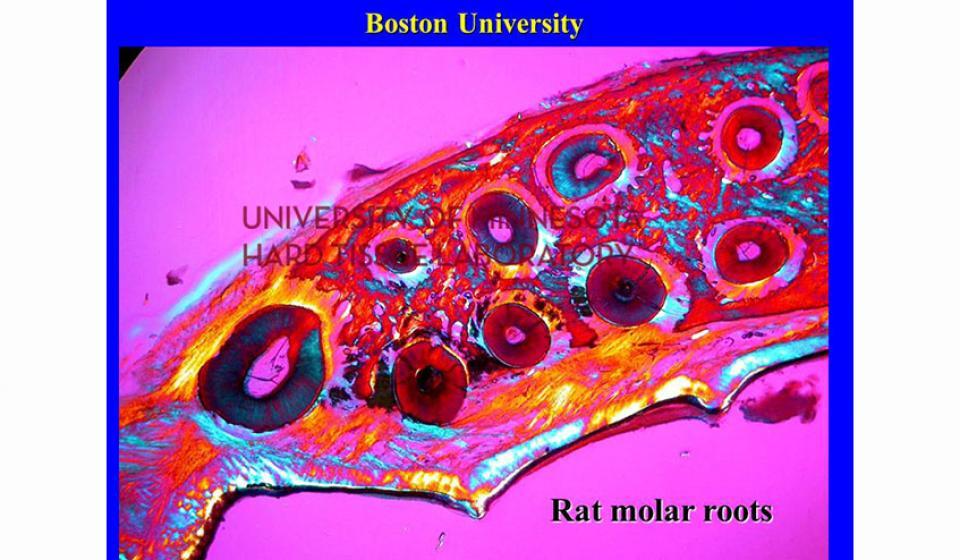
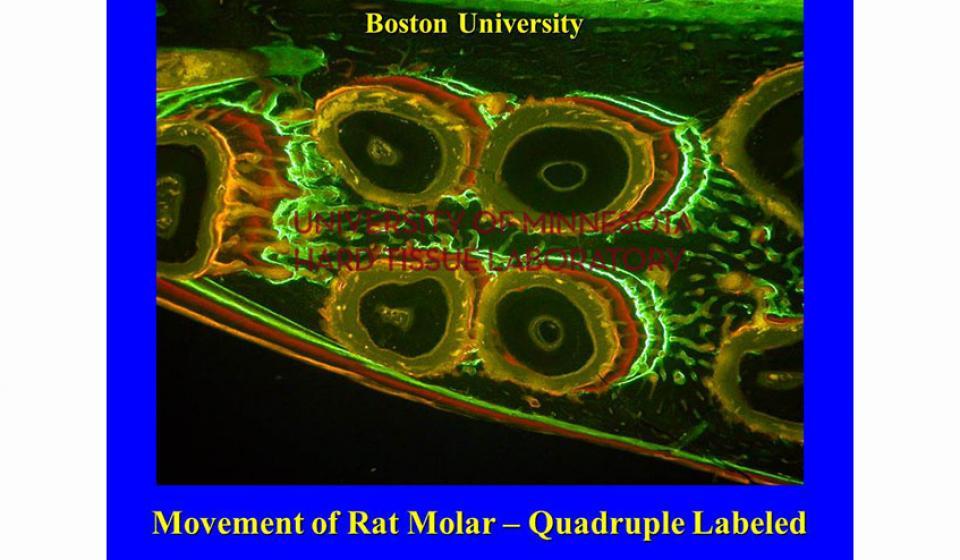
Welcome to the Hard Tissue Research Laboratory at the University of Minnesota School of Dentistry.
At the Hard Tissue Research Laboratory we investigate bone by using the Donath/EXAKT technique of undecalcified histotechnology. The majority of the research with which we're concerned involves implants. We also study various bone grafting materials and techniques. A new area of research is bone augmentation and growth enhancement using various bone promoting peptides and other natural or genetically engineered chemicals.
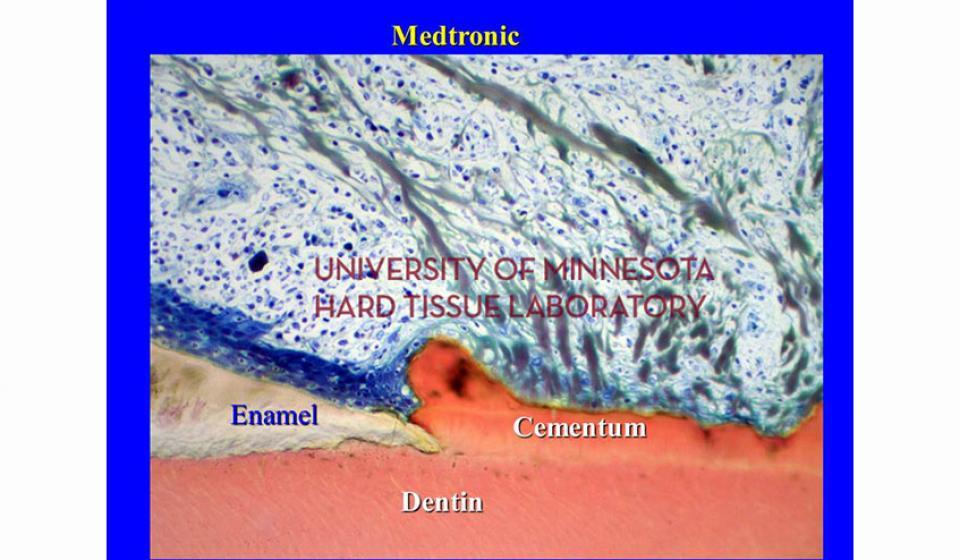
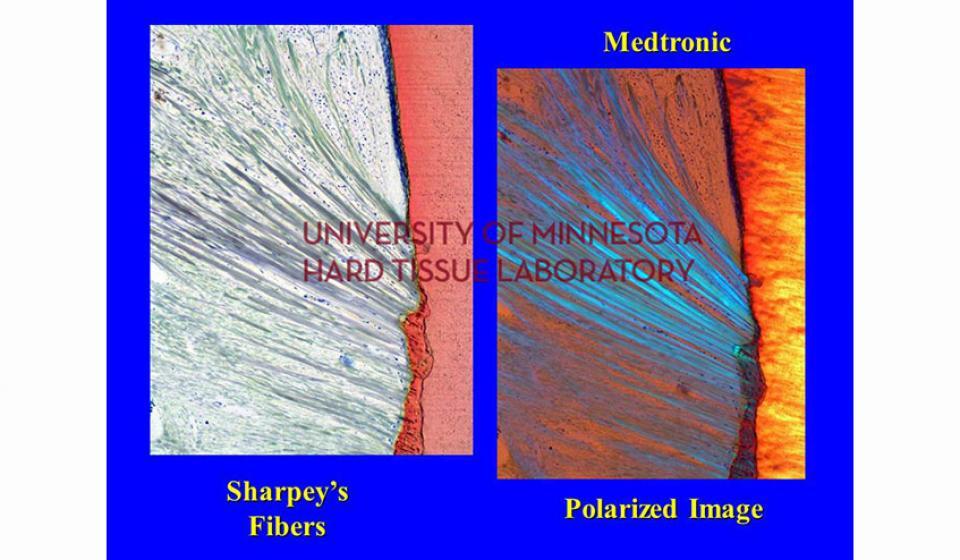
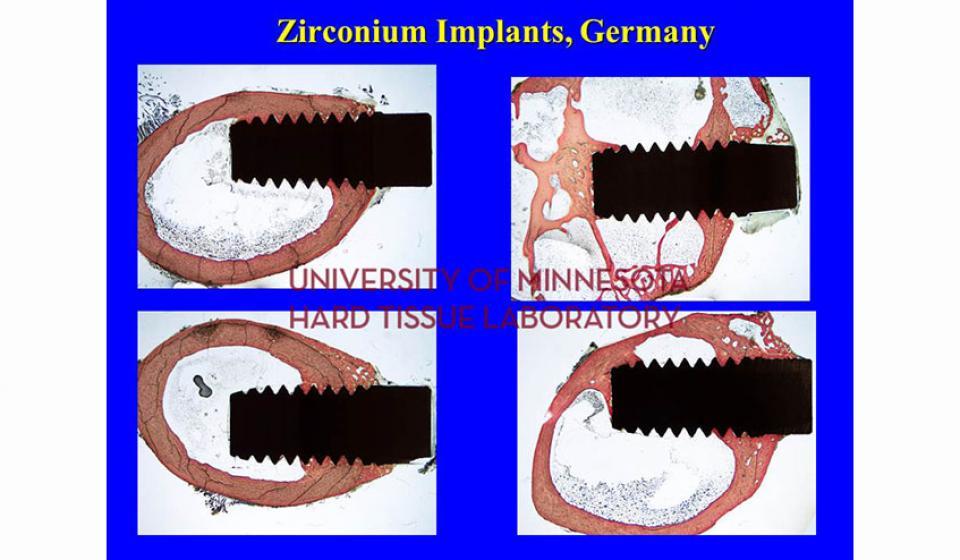
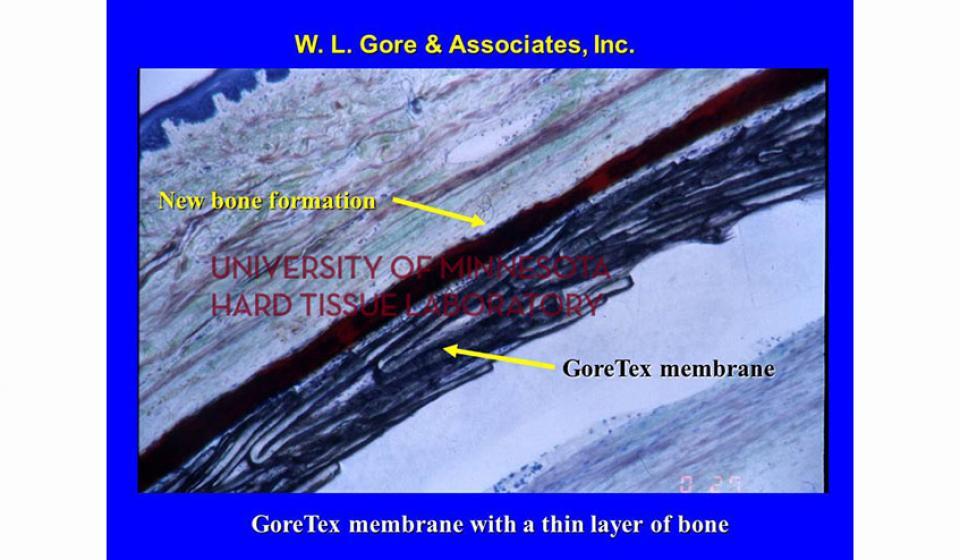
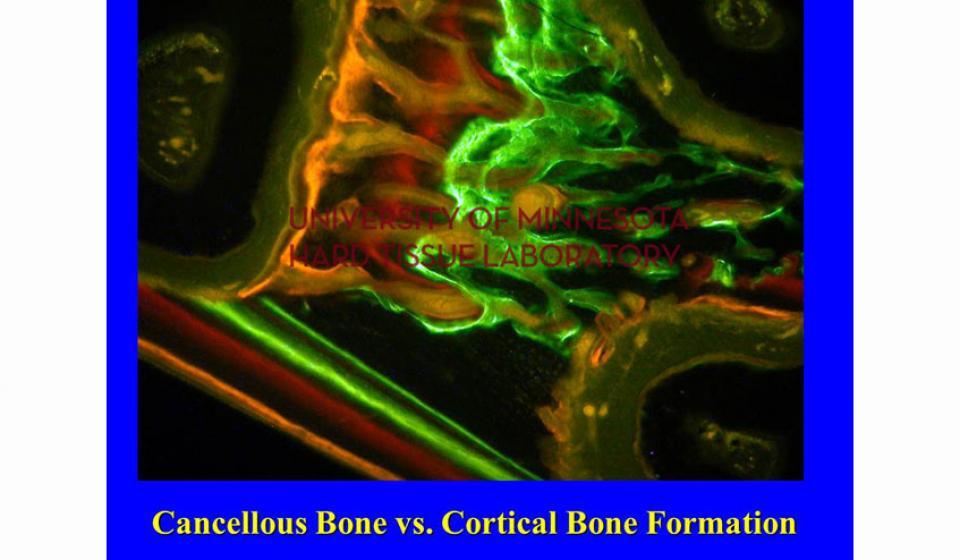
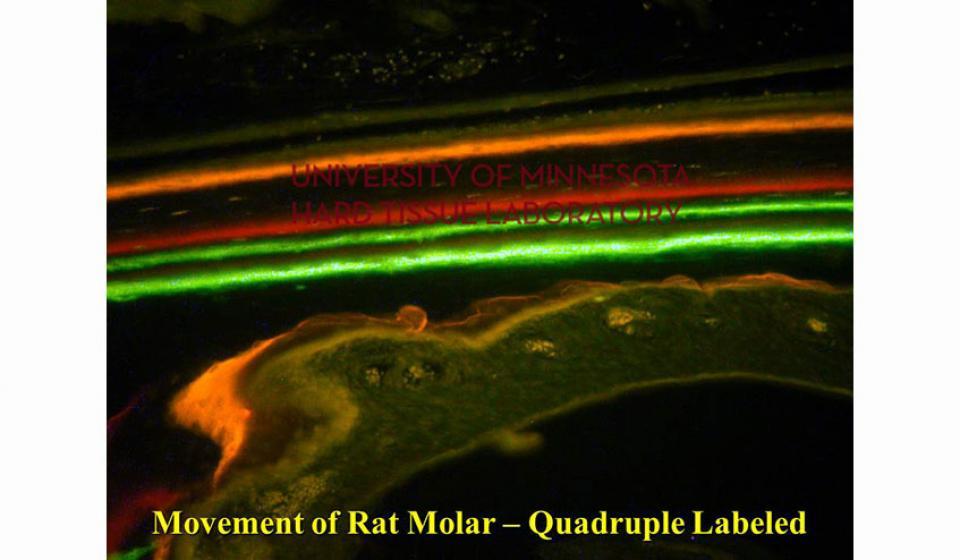
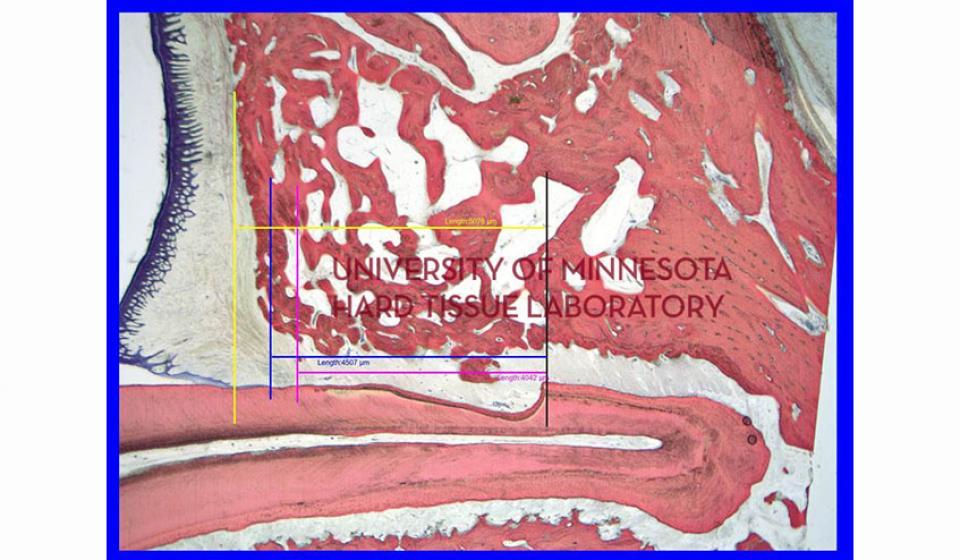
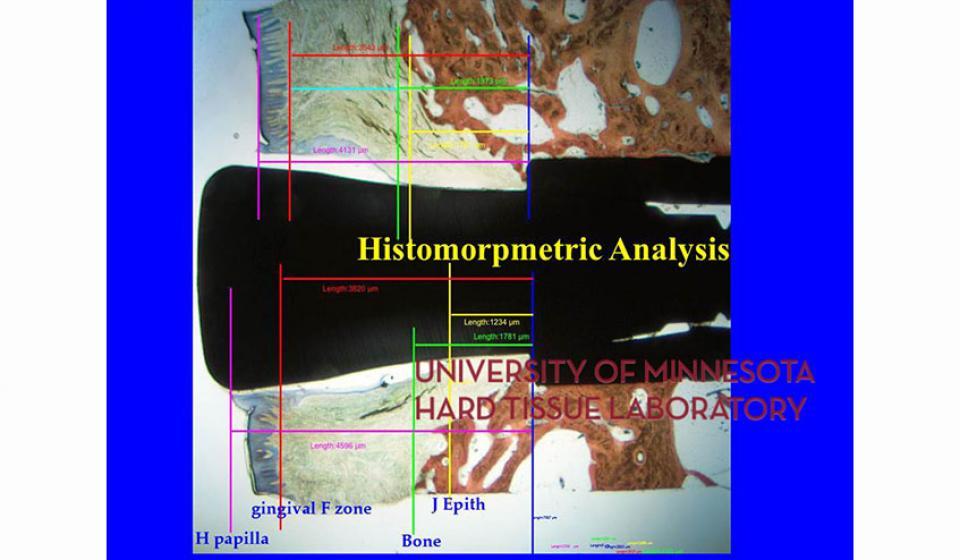
Bone specimens are dehydrated, infiltrated, and then embedded in a light curing resin. Very thin sections are possible and we generally use 35µm to 50µm sections for the final examination. The primary focus of many of our Hard Tissue Research Laboratory research projects is the implant/tissue interface. In this way, we are able to assess the osseointegration of the bone and metal surface. Final specimens are stained with various stains that emphasize the relative maturity of the bone as well as differentiate between vital, non-vital, calcified, and non-calcified bone elements. Computer assisted image analysis allows us to obtain histomorphometric information. This is especially useful in analyzing bone cores to determine the percentage of vital bone, non-vital grafted bone, and non-bone materials such as various types of hydroxyapatite (HA). Fluorescence microphotography of fluorochrome labelled bone specimens is also common.
Hard Tissue Research Laboratory Personnel
Hari S. Prasad
Hari Prasad is the Assistant Director of the Hard Tissue Research Laboratory, Division of Oral and Maxillofacial Pathology, and a Senior Research Scientist at the University of Minnesota School of Dentistry. Minneapolis, MN.
Hari Prasad graduated from University of Lucknow and King George’s Medical College, Lucknow, India. He completed his four years of studies in basic bone research at the University of Oklahoma under the guidance of Prof. Michael D. Rohrer. He has published over 76 research papers and he has been acknowledged in many scientific papers for his contributions. He is an author of the chapter Biology and Histology of Bone Grafts in the text Oral and Maxillofacial Surgery by Fonseca. He serves on Editorial Review Board to The Implant Dentistry Journal, flagship publication of the International Congress of Oral Implantologists (ICOI). He serves as a consultant to many universities for graduate research programs advising them on research design and protocol modifications.
Hari Prasad lectures extensively on bone, bone biomaterials, bone regeneration, dental implants and growth factors at national and international conferences. Over the past 24 years he has been active in research on dental implants, bone biomaterials, growth factors with collaborations within the US and around the world. He received his Fellowship and Mastership from the ICOI in 2000. He is a member of Sigma Xi, National Research Honorary Society, American Dental Education Association, Society of Dental Implantology, and the ICOI.
He is an Adjunct Assistant Professor at Augusta University, Dental College of Georgia and at the University of Oklahoma.
Hari Prasad has received several honors and awards including Excellence in Research Award for his contributions to orthopedic research in 1999. Ralph V. McKinney, Jr. Annual Award in Basic and Clinical Research in 2004 and 2008. from the ICOI, and First Place Award for Table Top and Poster Presentations at the ICOI World Congress XXVI in Vancouver, BC, Canada in 2009. Hari Prasad is also a Graduate of Plymouth Police Citizen Academy 2006, MN, US . He has traveled through 48 countries.
Photos
Who can I contact at the Hard Tissue Research Laboratory?
Call Hari Prasad at 612-626-2190 between 8:00 am and 5:00 pm Central Time, Monday through Friday or email at [email protected]. Feel free to leave a message if we're not available for the phone.
How should I prepare the specimens I harvest?
It is best to have as little bone around implants as possible given the constraints of the research project. Adequate fixation and infiltration of resin is enhanced by not having extra bone in the specimens. If the specimen contains a section of the mandible, it is best to remove the inferior cortical plate if possible without harming the implant. The clinical investigator should retrieve and prepare the specimens to as close as possible the ideal size before shipping them to the Hard Tissue Research Laboratory. When the specimens arrive in the laboratory, we will section the implants, buccal-lingually, mesio-distally, or in multiple cross sections, depending on the research protocol, prior to dehydration and infiltration Bone core specimens should be retrieved by as large a trephine as possible. The absolute minimum size of a core is 1mm in diameter and 2mm in length. Of course, the larger the core, the better the results. The bone core should be removed from the trephine prior to sending it to the lab.
Is a special fixative necessary?
The specimens should be fixed in 10% neutral buffered formalin, at least a ten-fold volume of fixative to volume of specimen. The specimens can be shipped in formalin or 70% alcohol. If shipping in alcohol, the specimens should fix at least 24-48 hours in formalin. If shipped overnight, the specimens can be simply wrapped in saturated gauze with the formalin or alcohol.
How should I package and label the specimens?
The specimens should be packaged individually and labeled well IN PENCIL on the outside and on a small piece of white paper on the inside of the container. Ink is not to be used. This primary container should be sealed so there can be no leakage. The primary container should be placed in a leak proof secondary container such as a high quality zip-lock bag which may or may not be labeled. The specimens should be packaged in a strong, crush proof box with enough absorbent packing material to soak up all the liquid should it leak. The box should not be able to be soaked with the liquid should it come out of the containers. Changes in air pressure during air shipment tends to cause formalin to leak out of containers which seem tight.
It is imperative that the specimens be well identified in the containers and also on an identification sheet. The information should include:
- Your identification number of the specimen
- Information on the specimen including
- Location
- Type of material
- Patient identification if appropriate
- Timing from placement to removal of specimen
- Any other pertinent information to aid in processing the specimen accurately
How should we ship the specimens to the laboratory?
The specimens should be shipped overnight, preferably with a shipper which can track the shipment all the way to the lab. It is best if one calls the Hard Tissue Research Laboratory at 612-626-2190 or Dr. Rohrer's office at 612-624-5478 to make sure someone will be at the laboratory to receive the shipment when it arrives so it can be immediately logged in and started on the dehydration/infiltration process.
The address for shipping specimens is:
Hard Tissue Research Laboratory
The University of Minnesota School of Dentistry
16-116 Moos Tower
515 Delaware St. S.E
Minneapolis MN 55455
How long does it usually take for the specimens to be processed?
The dehydration and infiltration process takes approximately 1 month. The time for the preparation of the slides depends on the number of specimens, what is required, and how busy the lab is at that time. In general, projects are completed between 60 and 120 days.
What about coverslipping the slides once we receive them from the Hard Tissue Research Laboratory?
These slides are not coverslipped when sent from our laboratory. They need to be coverslipped with oil before you view them microscopically or take pictures. Before viewing, use immersion oil or a very clear light machine oil to coverslip the slides. Do not try to evaluate or photograph the slides without coverslips.
What's the best way to store the slides for a long time?
For long term storage, the slides should be coverslipped with ample oil to prevent drying. Storage at room temperature in the dark is best to avoid fading of the stains.Anytime there is a bubble, dust or anything else in the coverslipping that inhibits photography, simply clean and recoverslip.
Coverslipping Alternative:
What we usually do at the Hard Tissue Research Laboratory is use immersion oil. As an alternative to placing a coverslip over the oil which often traps very small bubbles, I put the oil on and spread it around the area in which I'm interested and then, instead of putting a coverslip on, I use a coverslip as a squeegee and draw it across the slide firmly at about a 45 degree angle. This seems to leave a good layer of oil for viewing with few or no bubbles. Of course, it gathers dust immediately so I clean the slide carefully if I can't cover it up right away.
Hard Tissue Research Lab Scientific Articles
- Froum, S., Cho, S-C, Elian, N., Rosenberg, E., Rohrer, M.D., and Tarnow, D. Extraction Sockets and implantation of hydroxyapatites with membrane barriers: a histologic study. Implant Dent 2004;13:153-164
- Wikesjo, Ulf M. E., Qahash, Mohammed, Thomson, Robert C., Cook, Alonzo D., Rohrer, Michael D., Wozney, John M. & Hardwick, W. Ross. rhBMP-2 significantly enhances guided bone regeneration. Clinical Oral Implants Research15 (2), 194-204, March 2004
- Proussaefs, P., Lozada, J., Kim, J., and Rohrer, M.D. Repair of the perforated sinus membrane with a resorbable collagen membrane: a human study. Int J Oral Maxillofac Implants 2004;19:413-420
- Artzi, Z., Weinreb,M., Givol, N., Rohrer, M.D., Nemcovsky, C.E., Prasad, H.S., and Tal, H. Biomaterial Resorption Rate and Healing Site Morphology of Inorganic Bovine Bone and _-Tricalcium Phosphate in the Canine. A 24-month Longitudinal Histologic Study and Morphometric Analysis. Int J Oral Maxillofac Implants 2004;19:357-368
- Xiropaidis, A.V., Qahash, M., Lim. W.H., Shanaman, R.H., Rohrer, M.D., Sorensen, R.G., Li, X.J., Wozney, J.M., Wikesjo, U.M.E., Hall, J. Bone-implant contact at calcium phosphate coated and porous titani8um oxide (TiUnite) modified oral implants. Submitted Int'l J Oral Maxillo Impl, December 2003
- Artzi, Z., Givol, N., Rohrer, M., Nemcovsky, C., Prasad, H., and Tal, H. Qualitative and quantitative expression of bonine bone mineral in experimental bone defects. Part 1: description of a dog model and histological observations. J Periodontology 2003 Aug:74(8):1143-52
- Artzi, Z., Givol, N., Rohrer, M., Nemcovsky, C., Prasad, H., and Tal, H. Qualitative and quantitative expression of bovine bone mineral in experimental bone defects. Part 2: morphometric analysis. J Periodontology 2003 Aug:74(8):1153-60
- Hahn, J., Rohrer, M.D., and Tofe, A.J. Clinical, radiographic, histologic, and histomorphometric comparison of PepGen P-15 particulate and PepGen P-15 flow in extraction sockets: a same-mouth case study. Implant Dentistry 2003;12(2):170-4
- Silvera, M.R., Sheridan, P.J., Weaver, A., Rohrer, M.D., and Prasad, H. Effects of calcium sulfate and TGF-1 on bone regeneration at peri-implant defects. Submitted August 2003, J of Periodontology
- Benarroch, M., Otomo-Corgel, J., Rohrer, M., Pucher, J., Anson, D. and Prasad, H. Human histologic evaluation of periodontal regeneration following the treatment of periodontal osseous defects using a composite grft of calcium sulfate and demineralized freeze dried bone. Submitted August, 2003, Journal of Periodontology
- Proussaefs, P, Lozada, J., Kleinman, A., Rohrer, M.D., and McMillan, P.J. The use of titanium mesh in conjunction wit autogenous bone graft and inorganic bovine bone mineral (Bio-Oss) for localized alveolar ridge augmentation; a human study. Int J Periodontics Restorative dent 2003;23:185-195
- Wikesjo UME, Qahash M, Thomson RC, Cook AD, Rohrer MD, Wozney JM, Hardwick WR. Space-providing expanded polytetrafluoroethylene devices define alveolar augmentation at dental implants induced by recombinant human bone morphogenetic protein-2. Clinical Implant Dentistry and Related Research 2003; 5:112-123.
- Ko, C.C., Douglas, W.H., DeLong, R., Rohrer, M.D., Swift, J.Q., Hodges, J.S., An, K-N, and Ritman, E.L. Effects of implant healing time on cervical bone loss of a controlled-load dental implant. J Dent Res 82(8):585-591, 2003
- Froum, S., Cho, S., Elian, N., Rosenberg, E., Rohrer, M., and Tarnow, D. Histological comparison of healing extraction sockets following implantation of two different hydroxyapatites with two different membrane barriers in humans. A pilot study. Submitted to Journal of Periodontology 4/03
- Pogrel, M.A., Regezi, J.A., Fong, F., Hakim-Faal, Z., Rohrer, M.D., Tran, C., and Schiff, T. Effects of liquid nitrogen cryotherapy and bone grafting on artificial bone defects in minipigs: a preliminary study. Int J Oral Maxillofac. Surg. 31:296-302, 2002
- Proussaefs, P., Lozada, J., Kleinman, A., and Rohrer, M.D. The use of ramus autogenous block grafts for vertical alveolar ridge augmentation and implant placement: A pilot study. Int J Oral Maxillofac Implants 17:238-248, 2002
- Proussaefs, P, Lozada, J., Valencia, G, and Rohrer, M.D. Histologic evaluation of a hydroxyapatite onlay bone graft retrieved after 9 years: A clinical report. J Prosthet Dent 2002;87;481-4
- London, R.M. Roberts, F.A., Baker, D.A., Rohrer, M.D., and O'Neal, R.B. Histologic comparison of a thermal dual-etched implant surface to machined, TPS, and HA surfaces: Bone contact in vivo in rabbits. Int J Oral Maxillofac Implants, 2002; 17:369-376
- Wikesjo UME, Qahash M, Thomson RC, Cook AD, Rohrer MD, Wozney JM, Hardwick WR. rhBMP-2 significantly enhances guided bone regeneration. Clinical Oral Implants Research 2003; in press.
- Proussaefs, P, Lozada, J., Kleinman, A., and Rohrer, M.D. The use of intraorally harvested autogenous block grafts for vertical alveolar ridge augmentation. A human study. Int J Oral Maxillofac Implants 17(2):238-248, 2002
- Froum, S., Rohrer, M.D., Rosenberg, E., Cho, S.C., and Tarnow, D. Histological comparison of healing extraction sockets implanted with either bioactive glass or decalcified freeze-dried bone allograft. J Periodontol 2002 Jan. 73 (1) 94-102
- Evans, J, Sullivan, S., Rohrer, M.D., Prasad, H. Regeneration of peri-implant infraboney defects using BSM in white rabbits. Accepted for publication, Int J Oral Maxillofac Implants
- Counts, A.L, Rohrer, M.D.,Prasad, H.S., and Bolen, P. An assessment of root cementum in cleidocranial dysplasia. Angle Orthodontics, 2001, 71(4):293-298
- Proussaefs, P., Lozada, J, Kleinman, A., and Rohrer, M.D. A clinical and histologic evaluation of block onlay graft in conjunction with autogenous articulate and inorganic bovine mineral (Bio-Oss). A case report. Submitted March, 2001
- Proussaefs, P., Lozada, J, Kleinman, A., Rohrer, M.D. and McMillan P.J. The use of titanium mesh in conjunction with autogenous bone graft and inorganic bovine mineral (Bio-Oss) for localized alveolar ridge augmentation. A human study. Submitted 01/2001
- Proussaefs, P., Lozada, J, Valencia, G., and Rohrer, M.D. Histologic evaluation of a hydroxyapatite (Interpore 200) onlay bone graft retrieved after 10 years. A case report. Submitted 12/2000
- Goradia, V.K., Rochat, M.C., Grana, W.A., Rohrer, M.D., and Prasad, H.S. Tendon-to-bone healing of a semitendinosus tendon autograft used for ACL reconstruction in a sheep model. Am J Knee Surg. 2000;13:143-151
- Tatakis, D.N., Koh, A., Jin, L., Wozney, J.M., Rohrer, M.D., and Wikesjo, U.M.E. Peri-implant bone regeneration using recombinant human bone morphogenetic protein-2 in a canine model: a dose-response study. J Periodont Res 2002; 37:93-100
- Krauser, J.T., Rohrer, M.D., and Wallace. S.S. Human histologic and histomorphometric analysis comparing OsteoGraf/N with PepGen P-15 in the maxillary sinus elevation procedure: a case report. Implant Dentistry 9(4):298-301, 2000
- Proussaefs, P.T., Tatakis, D.N., Lozada, J., Caplanis, N. and Rohrer, M.D. Histologic evaluation of hydroxyapatite-coated root form implants retrieved after 7 years in function. A case report. Int J Oral Maxillofac Implants 2000; 15:438-443
- Nadon, N.L., Doersen, C-J., Lade, D.A., Medina, K.,.Thompson, L., Rohrer, M.D., and Gimble. Multiple cellular phenotypes in an insertional mutation in mouse affecting bone development. Calcified Tissue International, 2000, 67:449-454
- Tarnow, D.P., Wallace, S.S., Froum, S., Rohrer, M.D. Histologic and clinical comparison of bilateral sinus floor elevations with and without barrier membrane placement in 12 patients: part 3 of an ongoing prospective study. Int J Perio and Rest Dent 2000, 20(2):117-125
- Wu, X, Peters, J.M., Gonzalez, F.J., Prasad, H.S., Rohrer, M.D., Gimble, J. M. The frequency of stromal lineage colony forming units in the bone marrow of peroxisome proliferator-activated receptor-_-null mice. BONE, 26:1:21-26, January, 2000
- Rohrer, M.D., Sobszak, R., Prasad, H.S. and Morris, H.F. Postmortem Histologic Evaluation Of Mandibular Titanium And Maxillary Hydroxyapatite-Coated Implants In Situ For 85 And 38 Months. Int J Oral Maxillofac Implants 14:579-586, 1999
- Akimoto, K., Becker, W., Persson, R., Baker, D., Rohrer, M., and O'Neal, R. Evaluation of titanium implants placed into simulated extraction socket: a study in dogs. Int J Oral Maxillofac Implants 14:351-360, 1999
- Froum, S.J., Tarnow, D.P., Wallace, S.S., Rohrer, M.D., and Cho, S.C. Sinus floor elevation utilizing anorganic bonine bone matrix (OsteoGraf/N) with and without autogenous bone: a clinical, histologic, radiographic, and histomorphometric analysis: Part 2 of an ongoing prospective study. Int J Periodont Rest Dent 1998, 18(6):528-543
- Rohrer, M.D. and Prasad, H.S. The hard tissue research laboratory; a unique research asset at the University of Oklahoma College of Dentistry. Oklahoma Dental Association Journal 89:36-38, 1998
- Johnson, M.W., Sullivan, S.M., Rohrer, M.D., Collier, M. Regeneration of peri-implant infrabony defects using perioglas: A pilot study in rabbits. Int J Oral Maxillofac Implants 12:835-839, 1997
- Dominici, J.T., Olson, J., Rohrer, M.D., and Morris, H. Post-mortem histologic evaluation of hydroxyapatite-coated cylinder and titanium alloy basket implants in situ for 37 months in the posterior mandible. Implant Dentistry 6:215-222, 1997
- Sigurdsson, T.J., Fu, E., Tatakis, D.N., Rohrer, M.D., and Wikesjo, U.M.E. Bone morphogenetic protein-2 enhances peri-implant bone regeneration and osseointegration. Clin Oral Impl Res 8:367-374, 1997
- Rayan GM, Murray D, Chung KW, Rohrer MD: The extensor retinacular system at the metacarpophalangeal joint: anatomical and histological study. Journal of Hand Surgery (British and European Volume)22B:5:585-590, 1997
- Hanisch, O., Tatakis, D.N., Boskovic, M.M., Rohrer, M.D., and Wikesjo, U.M.E. Bone formation and reosseointegration in peri-implantitis defects following surgical implantation of rhBMP-2. Int J Oral Maxillofac Implants 12:604-610, 1997
- Hanisch, O.H., Tatakis, D.N., Rohrer, M.D., Wohrle, P.S., Wozney, J.M., and Wikesjo, U.M.E. Bone formation and osseointegration stimulated by rhBMP-2 following subantral augmentation procedures in nonhuman primates. Int J Oral Maxillofac Implants 12:785-792, 1997
- Caplanis, N.C., Sigurdsson, T.J., Rohrer, M.D. and Wikesjo, U.M.E. Effect of allogeneic freeze-dried demineralized bone matrix on guided Bone regeneration in supraalveolar peri-implant defects in dogs. Int J Oral Maxillofac Implants 12:634-642, 1997
- Desai, S., Johnson, D.L., Howes, R.I., and Rohrer, M.D. Changes in the Rabbit Temporomandibular Joint Associated with Posterior Displacement of the Mandible. International Journal of Prosthodonitcs 9:46-57, 1996
- Sinha, P.K., Rohrer, M.D., Nanda, R.S., and Brickman, C.D. Interlayer formation and its effect on debonding polycrystalline alumina orthodontic brackets. American Journal of Orthodontics and Dentofacial Orthopedics 108;5: 455-463, 1995
- Pimenta J, Rohrer MD, Castro F, Peres F: Titanium and Osteointegration - Preliminary Study. Oklahoma Dental Association Journal 85: 30-34, 1995.
- Patterson MK, Bulard RA, Larsen C, Rohrer MD: Imtec Implants: Clinical Evaluation of 120 Patients: 548 Implants. Oklahoma Dental Association Journal 85: 12-15, 1995.
- Rohrer, MD, Bulard, RA, Patterson, MK: Maxillary and Mandibular Titanium Implants One Year After Surgery: Histologic Examination in a Cadaver. Int J Oral Maxillofac Implants, 10(4)466-473, 1995.
- Hough, JVD, Wilson, N, Dormer, KJ, Rohrer, MD: The Audiant Bone Conductor: Update of Patient Results in North America. American Journal of Otology 15(2)189-197, March 1994.
- Paranjpe, M.G., Sundeep Chandra, A.M., Qualls, C.W.,Jr., McMurry, S.T., Rohrer, M.D., Whaley, M.M. and Lochmiller, R.L. Fluorosis In A Wild Cotton Rat (Sigmodon hispidus) Population Inhabiting A Petrochemical Waste Site. Toxicologic Pathology 22(6)569-578, 1994.
- Collier, M.A., Haugland, L.M., Bellamy, J., Johnson, L.L., Rohrer, M.D., et al: Effects of Holmium: YAG Laser on Equine Articular Cartilage and Subchondral Bone Adjacent to Traumatic Lesions: A Histopathological Assessment. Journal of Arthroscopic and Related Surgery 9(5)536-545, 1993.
- Larsen, P.E., Stronczek, M.J., Beck, F.M., and Rohrer, M.D. Osteointegration of Implants in Radiated Bones With and Without Adjunctive Hyperbaric Oxygen. Journal of Oral and Maxillofacial Surgery, 51:280-287, 1993
- Callan, D.P. Rohrer, M.D. Use of Bovine-Derived Hydroxyapatite in the Treatment of Edentulous Ridge Defects - A Human Clinical and Histologic Case Report. Journal of Periodontology 64:6, 1993.
- Rohrer, M.D. and Schubert, C.C. The cutting-grinding technique for histologic preparation of undecalcified bone and bone-anchored implants: improvements in instrumentation and procedures. Oral Surgery Oral Medicine Oral Pathology. 74:73-78, 1992.
- Rohrer, M.D. and Schubert, C.C. A Cutting-Grinding Technique for the Preparation of Undecalcified Hard Tissue Materials. Biomaterials Forum 13: 21, 1991
- Donath, K., Rohrer, M.D., Hoermann, K. Mobile and Immobile Hydroxylapatite Integration and Resorption and Its Influence on Bone. Oral Implantology, 13(1):120-127, 1987.
- Donath, K., Rohrer, M.D., and Beck-Mannagetta, J. Histological Evaluation of a Mandibular Cross Section One Year After Augmentation with Hydroxylapatite Particles. Oral Surgery Oral Medicine Oral Pathology, 63:651-655, June, 1987.